Notification to consumers and HCPs
LUCIRA® by Pfizer COVID-19 & Flu Home Test and Lucira® by Pfizer for COVID-19 & Flu Test (For use under Emergency Use Authorization (EUA) only)
The notification is to inform consumers and HCPs that certain lots of the test kits include nasal swabs that expired before the labeled expiration date printed on the outer test package. The impacted kits expire between February and June 2025, while the nasal swab expired in January 2025.Click here to learn more
Read the Emergency Use Authorization for LUCIRA® by Pfizer COVID-19 & Flu Test


For ages 2 and up
LUCIRA® by Pfizer COVID-19 & Flu Test can help with timely and accurate* detection of COVID-19, Flu A, and Flu B using a single swab1
The first 3-in-1, single-use, molecular test for use at the point of care1,2
Emergency Use Authorization
The LUCIRA® by Pfizer COVID-19 & Flu Test has not been FDA cleared or approved, but has been authorized for emergency use by FDA under an EUA for use by authorized laboratories. This product has been authorized only for the detection and differentiation of nucleic acid from SARS-CoV-2, influenza A, and influenza B, not for any other viruses or pathogens. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
See the technology behind the test.
LUCIRA® by Pfizer COVID-19 & Flu Test is a portable device that can be used in a variety of point-of-care settings.1
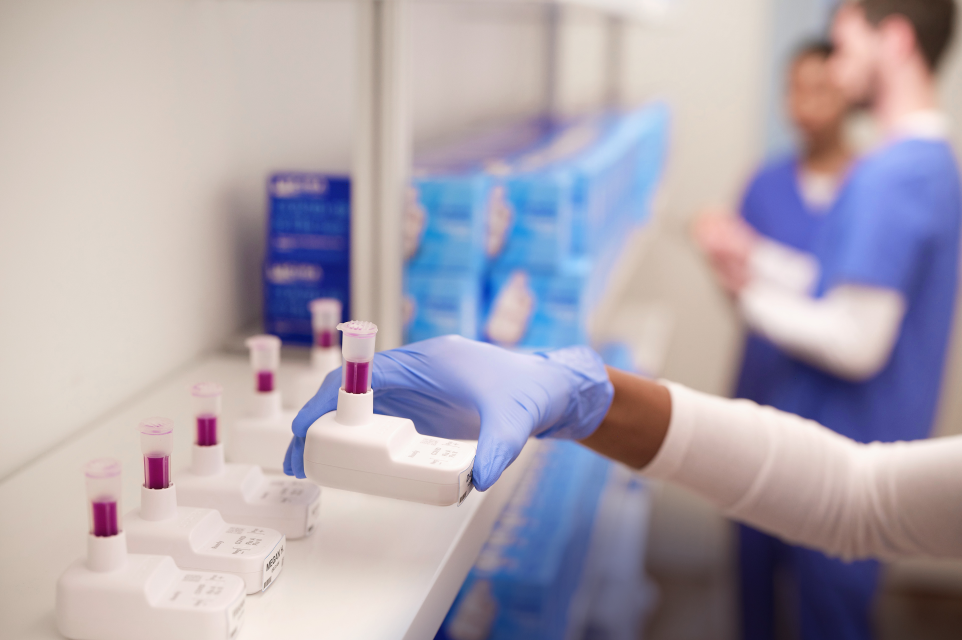

Timely diagnosis of COVID-19 and flu for symptomatic patients
Using a molecular multiplex test can help facilitate early diagnosis and streamline patient care.3,4
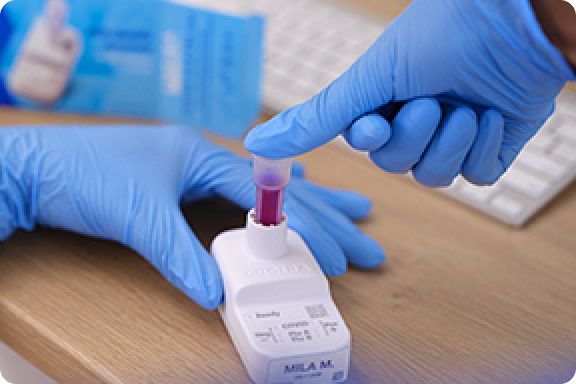
PCR-quality accuracy* with results in 30 minutes
LUCIRA® by Pfizer COVID-19 & Flu Test uses molecular technology to deliver lab-quality accuracy* to your practice.1

*The LUCIRA® by Pfizer COVID-19 & Flu Test provides a level of accuracy comparable to highly sensitive lab-based polymerase chain reaction (PCR) tests. Negative results do not preclude SARS-CoV-2, influenza A, and/or influenza B infection and should not be used as the sole basis for patient management decisions.1
References: 1. LUCIRA® by Pfizer COVID-19 & Flu Test. Instructions for Use. Pfizer Inc; 2023. 2. LUCIRA® by Pfizer COVID-19 & Flu Test letter of authorization. U.S. Food & Drug Administration. Published June 15, 2023. Accessed September 14, 2023. https://www.fda.gov/media/163455/download 3. CDC’s influenza SARS-CoV-2 multiplex assay. Centers for Disease Control and Prevention. Updated November 14, 2022. Accessed April 16, 2024. https://www.cdc.gov/coronavirus/2019-ncov/lab/multiplex.html 4. Clark TW, Lindsley K, Wigmosta TB, et al. Rapid multiplex PCR for respiratory viruses reduces time to result and improves clinical care: results of a systematic review and meta-analysis. J Infect. 2023;86(5):462-475. doi:10.1016/j.jinf.2023.03.005